Understanding how to draw a Lewis structure is essential for students studying chemistry. A Lewis structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist in the molecule. By following a few simple steps, you can easily draw a Lewis structure for any molecule.
It is important to remember that a Lewis structure is a two-dimensional representation of a molecule, and it does not accurately represent the three-dimensional shape of the molecule. However, it is a useful tool for understanding the bonding and electron distribution in a molecule.
Steps to Draw a Lewis Structure
1. Determine the total number of valence electrons in the molecule. This can be done by adding up the valence electrons of each atom in the molecule. Remember to take into account any charges on the atoms.
2. Determine the central atom in the molecule. The central atom is usually the least electronegative atom in the molecule, or the atom that can form the most bonds. Hydrogen and halogens are never central atoms.
3. Connect the central atom to the other atoms in the molecule using single bonds. Remember that each bond represents two electrons.
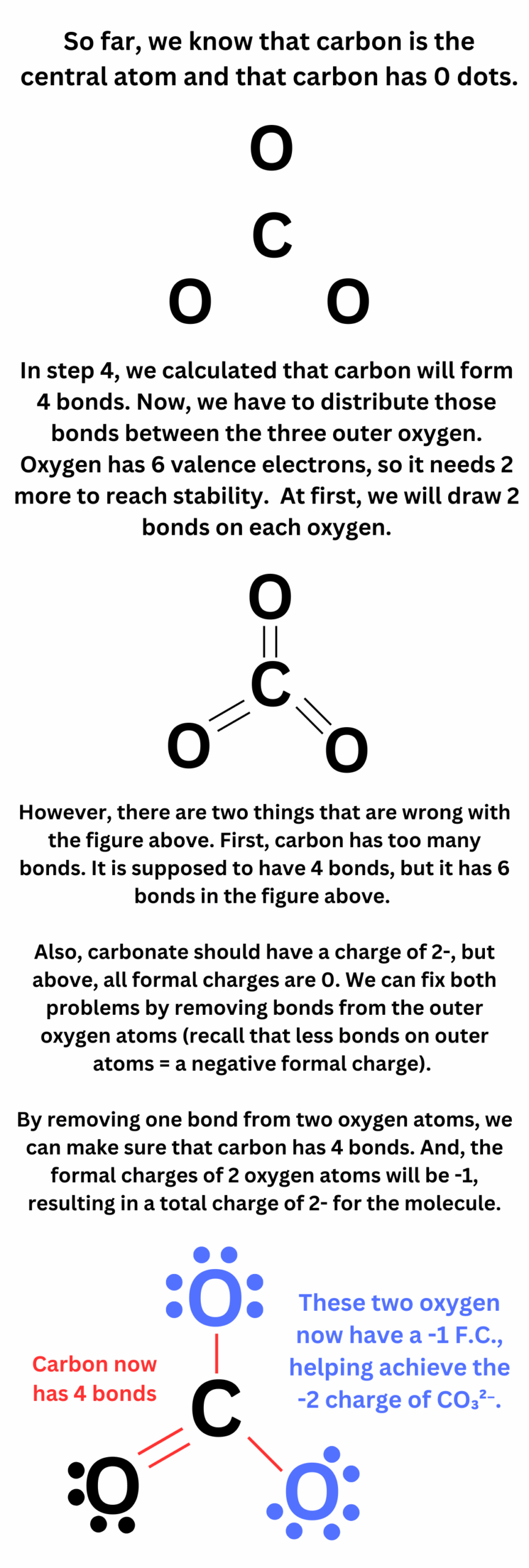
4. Distribute the remaining electrons as lone pairs on the outer atoms. Keep in mind that each atom (except hydrogen) should have an octet of electrons, which means it should have eight electrons in its valence shell.
5. If there are still electrons left after all the atoms have an octet, place them on the central atom. The central atom can exceed an octet if it is in the third row of the periodic table or below.
By following these steps, you can draw a Lewis structure for any molecule and gain a better understanding of its bonding and electron distribution. Practice drawing Lewis structures for different molecules to improve your skills in chemistry.
In conclusion, drawing a Lewis structure is an important skill for students studying chemistry. By following a few simple steps, you can easily draw a Lewis structure for any molecule and gain insights into its bonding and electron distribution. Remember to practice drawing Lewis structures regularly to improve your understanding of chemical compounds.